iVention Pharmaceutical
Solution
Powering Secure & Compliant Pharmaceutical Lab
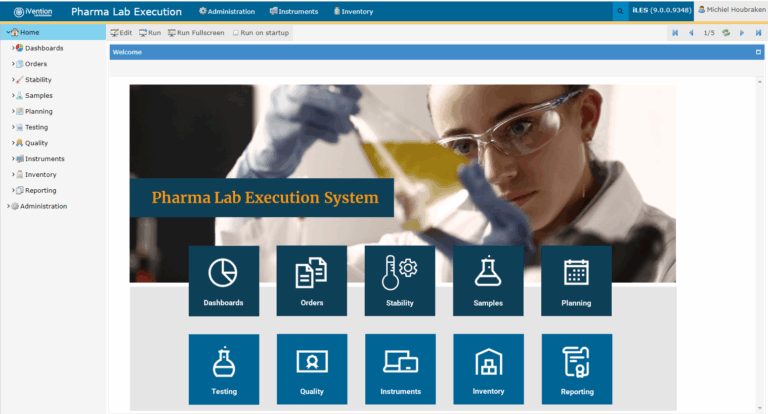
iLES Pharmaceutical LIMS is an advanced, all-in-one Laboratory Execution System (LES) and Laboratory Information Management System (LIMS) designed to meet the complex and regulated needs of pharmaceutical labs. It addresses challenges like manual data entry, fragmented workflows, and compliance by automating processes, improving traceability, and supporting international regulations such as FDA 21 CFR Part 11 and EU Annex 11.
This fully web-based, cloud-deployable platform adapts to various lab functions from QC to R&D—while maintaining centralized control and standardized workflows. Its entity-based architecture ensures full traceability from sample login to result approval.
iLES streamlines lab operations by integrating sample tracking, instrument data capture, test planning, and result verification into one unified system. Automation replaces repetitive tasks with intelligent workflows, improving productivity and data reliability. Built-in audit trails, electronic signatures, and version control keep labs inspection-ready.
Real-time dashboards and reports provide transparency and actionable insights, enabling faster decision-making and better collaboration. With iLES Pharmaceutical LIMS, pharmaceutical labs enhance efficiency, maintain compliance, and drive continuous improvement.
Download Brochure
You are currently viewing a placeholder content from HubSpot. To access the actual content, click the button below. Please note that doing so will share data with third-party providers.
More InformationiLES Pharmaceutical LIMS is an advanced, all-in-one Laboratory Execution System (LES) and Laboratory Information Management System (LIMS) designed specifically to meet the complex and regulated needs of the pharmaceutical industry. In an environment where data integrity, compliance, and efficiency are critical, iLES offers a smarter and more streamlined approach to laboratory operations. Traditional pharmaceutical labs often struggle with manual data entry, disconnected systems, and inefficient workflows. iLES Pharmaceutical LIMS addresses these challenges head-on by automating processes, improving traceability, and simplifying compliance with international regulatory standards such as FDA 21 CFR Part 11, EU Annex 11, and GxP.
This innovative platform is fully web-based and can be deployed easily via the Cloud, eliminating the need for on-premise infrastructure or complex installations. With its flexible architecture, iLES adapts to the unique requirements of different pharmaceutical labs—whether in QC, R&D, or stability testing—while maintaining centralized control and standardization across sites. Its entity-based structure enables the creation of configurable workflows that reflect the way each lab operates, offering full traceability from sample login to final result approval.
iLES Pharmaceutical LIMS empowers laboratories to digitize and manage every aspect of the lab workflow. From automated sample tracking and integrated instrument data capture to test planning, exception handling, and result verification, the system brings together all lab functions into one unified digital environment. This integration reduces the need for paper-based documentation and manual transcription, significantly lowering the risk of human error while improving turnaround times.
Automation is a core strength of iLES. It replaces repetitive, manual tasks with intelligent, rule-based workflows. For instance, test calculations, pass/fail logic, and results validation can be set up to run automatically. Alerts and notifications ensure that issues are identified and addressed promptly. This not only improves lab productivity but also enhances overall data reliability and regulatory preparedness. The system includes built-in tools for audit trails, electronic signatures, and version control, helping labs stay inspection-ready at all times.
One of the most powerful advantages of iLES Pharmaceutical LIMS is its ability to provide real-time access to operational data and performance metrics. Dashboards and reports offer clear visibility into lab activity, enabling better planning, faster decision-making, and improved collaboration across departments or sites. This transparency is essential for pharmaceutical companies seeking to optimize efficiency, meet compliance expectations, and drive continuous improvement.
Key features included in the iLES Pharmaceutical
- Sample Management
- Order Management
- Batch & Recipe Management
- Multilevel Specifications
- Scheduling & Planning (Resources, Tests, Sampling)
- Stability Studies
- Configurable Forms & Notebooks
- Method Development and Validation
- Test & Method Execution
- External Testing
- Standard Operation Procedure Compliance
- Multilevel Results Review
- Out-of-Spec investigations
- Control Charts
- Environmental Monitoring
- System Integration and Connectivity (ERP, CRM, MES, CDS, ...)
- Unidirectional or bidirectional Instrument Interfacing
- Instrument Management
- Inventory Management (Chemicals/References/Standards)
- Collaboration Portal
- QA/QC Management
- Qualifications & Training Records
- Incident and Change Control
- Compliance and Good Data Governance (GMP, ISO 17025 and FDA 21 CFR Part 11)
- Electronic Records retention and archival
- Powerful Workflow Management (ProcessStations, Visual Image Maps)
- Labelling Design (2D/3D/QR) & Scanning
- Reporting & Data Analytics
- Real-time Dashboards
- Global Search and Data Retrieval
- Secure System Access and Controls
- Authorization Matrix
- Lab Accreditation Management
- Document Management
- Chain of Custody tracking & Recording
Find out more
Contact us to share your plans and/or schedule a free demo. we are ready to take up your challenge!
iVention - More than a LIMS
iVention provides laboratories in a wide range of industries with state-of-the-art industry solutions that combine LIMS, LES, ELN, and SDMS functionality in a single informatics system. The powerful and flexible iLES platform is 100% web-based and can be easily deployed via the cloud and used on any (mobile) device. With iLES, labs get real-time information, ensure data integrity and regulatory compliance, while saving time and money through efficient process automation and less manual data processing. Since the successful launch of iLES in 2009, iVention has grown at a breathtaking pace and established itself as a consolidated player in the global informatics market.